认 证:工商信息已核实
访问量:752950

上海胤煌科技有限公司-伞棚灯、不溶性微粒 2020-02-17 点击3097次
What is Zeta Potential / 什么是Zeta电位?
1. 什么是Zeta电位?
Zeta potential is an electrostatic potential that exists very near the surface of particles suspended in liquids1. Zeta potential (ζ) is responsible for particle-particle repulsion forces in colloidal suspensions and thus can be used to predict colloid stability against particle aggregation. Figure 1 illustrates a particle suspended in a liquid along with various notional regions around it. The“slipping plane” or “shear plane” is where Zeta potential is located versus the potential in the bulk solution. Within this slipping plane, the liquid is bound to the particle while it moves freely outside this boundary. The net potential far from the particle (in the bulk of the liquid) is zero.
Zeta电位是液体中悬浮的粒子很接近表面位置的静电势1。Zeta电位(ζ)是由胶体中粒子与粒子间的相互作用造成的,因此它可以用来预测胶体体系里粒子聚集的稳定性。图1显示了悬浮在液体中的粒子及其周围的各种概念区域。Zeta电位指的是液体中滑动面或者剪切面的电位。在这个滑动平面内,当液体在这个边界外自由运动时,它与粒子结合在一起。远离粒子的净电势(在液体中)为零。
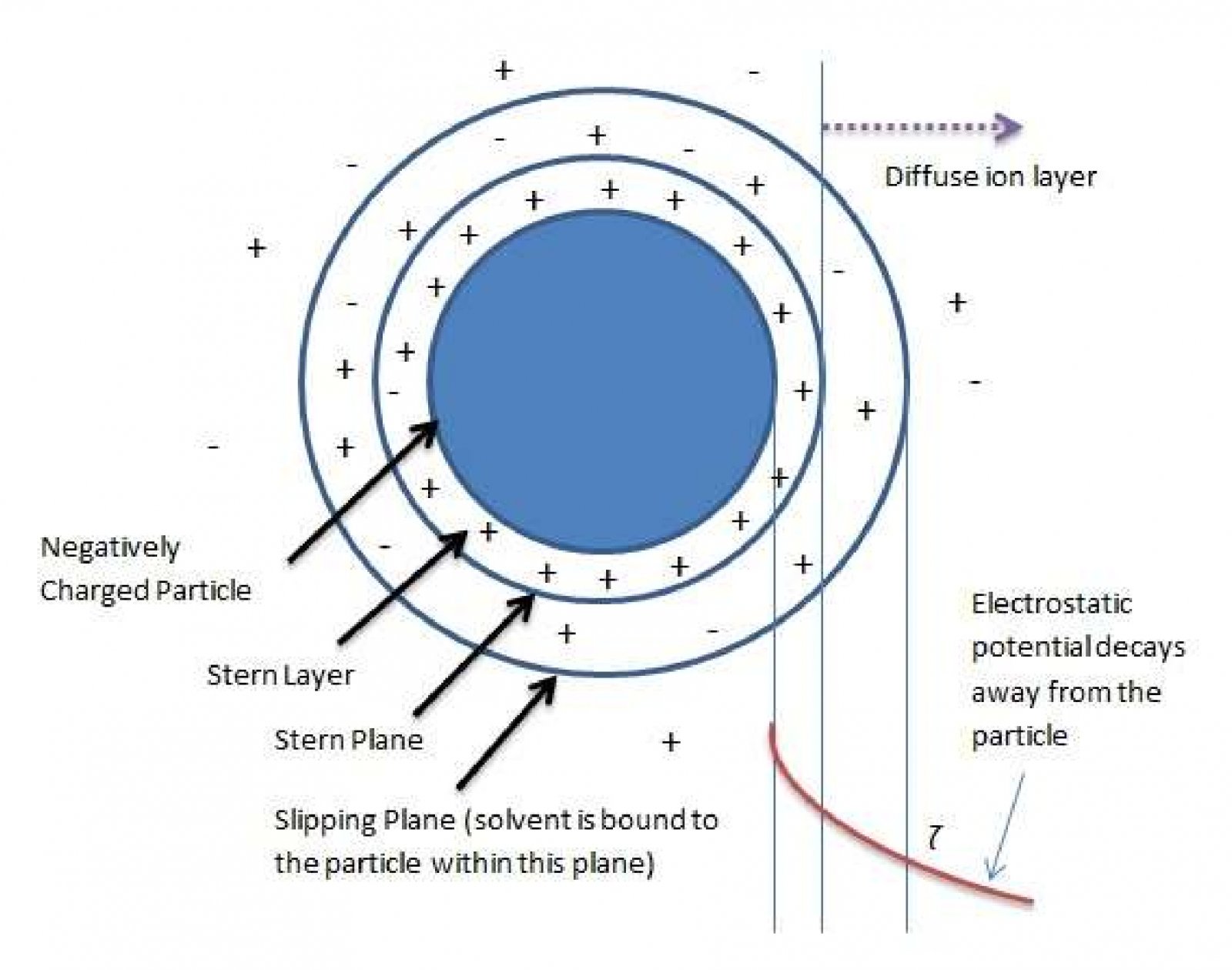
Figure 1. A negatively charged particle suspended in a liquid. Notional boundaries are shown.
图1悬浮在液体中的带负电的粒子及其周围的各种概念区域
PS:这个是另外的一种说法,但是要描述的内容是一样的;
由于分散粒子表面带有电荷而吸引周围的反号离子,这些反号离子在两相界面呈扩散状态分布而形成扩散双电层。根据Stern双电层理论可将双电层分为两部分,即Stern层和扩散层。Stern层定义为吸附在电极表面的一层离子(IHP or OHP)电荷中心组成的一个平面层,此平面层相对远离界面的流体中的某点的电位称为Stern电位。稳定层(Stationary layer) (包括Stern层和滑动面slipping plane以内的部分扩散层) 与扩散层内分散介质(dispersion medium)发生相对移动时的界面是滑动面(slipping plane),该处对远离界面的流体中的某点的电位称为Zeta电位或电动电位(ζ-电位)。
2. 测Zeta电位为什么不能稀释?
In aqueous media, Zeta potential is typically generated as the ions on the particle surface dissociate, leaving a net electric charge near the surface surrounded by a cloud of counter-ions. Various types of ions can diffuse in and out through the slipping plane which allows Zeta potential to vary depending on the ion composition in the liquid such as pH. Ions may also participate in chemical reactions within the slipping plane which can affect the Zeta potential. Sample dilution can significantly shift the Zeta potential as ions may adsorb or desorb from the particle. Thus, Zeta potential can be positive or negative, or zero (Iso-Electric Point, IEP) depending on the liquid (solvent) pH or ion type and concentration.
在水相介质中,Zeta电位通常是由于粒子表面的离子离解而产生的,在表面附近留下一个被反离子云包围的净电荷。各种类型的离子可以通过滑动面扩散进来和出去,滑动面允许Zeta电位根据液体中的离子组成而变化,例如pH值。离子也可以通过参与滑动面内的化学反应,从而影响Zeta电位。样品稀释可以显著地改变Zeta电位,因为离子可以吸附或者解析颗粒。因此,Zeta电位可以是正的或负的,也可以是零(等电点,IEP),这取决于液体(溶剂)的pH值或离子的类型和浓度。
3. 测量Zeta电位的方法
Particle-filtration systems may benefit from low Zeta potential levels as aggregated particles are easier to remove. Most other colloidal systems require higher Zeta potentials, e.g. over +/- 20 millivolts in order to maximize shell life. Coatings tend to be more efficient when the particles and coated surface have opposite polarities. Zeta potential normally cannot be directly measured. For example, one cannot place a voltmeter probe against a particle surface in order to measure its surface potential. Instead, Zeta potential is calculated from electrophoretic measurements which measure particle velocity under an applied electric field, i.e. make the particles move and measure their particle mobility (see ). Thus, the calculated Zeta potential depends on the theory used in these computations to relate particle mobility to Zeta potential. An alternative measurement for large particles or surfaces is to move the liquid against stationary particles, fibers, or surfaces and measure the resulting streaming potential。
颗粒过滤系统可能受益于较低的Zeta电位水平,因为聚集颗粒更容易去除。大多数其他胶体系统需要较高的Zeta电位,例如超过+/- 20毫伏,以最大限度地提高壳体寿命。当颗粒和涂层表面具有相反的极性时,涂层往往更有效。Zeta电位通常不能直接测量。例如,不能将伏特计探头靠在粒子表面上以测量其表面电位。相反,Zeta电位是通过电泳测量来计算的,电泳测量是在外加电场下测量粒子速度,也就是通过粒子移动并测量其粒子迁移率(见)。因此,计算出的Zeta电位取决于这些计算中使用的理论,即粒子迁移率与Zeta电位的关系。另一种测量大颗粒或表面电位的方法是将液体移到静止的颗粒、纤维或表面上,然后测量产生的流动电位。
4. 电位滴定法测量Zeta电位和PH的关系
Potentiometric Titrations are useful for determining a sample's IEP. As mentioned above, the IEP may be desirable or otherwise. Potentiometric Titration plots may display plateau regions for Zeta potential vs. pH. Such measurements enable manufacturers to optimize use of acids or bases for transportation and storage. Figure 2 shows a potentiometric titration on a Ludox-TM silica sample by automatic addition of 1N HCl. This titration was performed automatically by a Zeta-APS, Zeta Acoustic Particle Sizer, instrument from Matec Applied Sciences, Northborough, MA USA 3. Figure 2 shows that below pH 4, the Zeta curve approaches a plateau region while Conductivity increases more rapidly. This suggests that the silica particles are becoming saturated with H+ ions as the pH is lowered. Conductivity increases more rapidly as more of these H+ ions stay in the continuous water solvent as opposed to diffusing through the slipping plane toward the particle surface.
电位滴定法可用于测定样品的等电点。如上所述,IEP可能是可取的或者相反。电位滴定图可以显示Zeta电位与pH值之间的关系变化。这样的测量使制造商通过酸或碱的使用,优化产品的运输和储存。图2显示在Ludox TM二氧化硅样品上自动添加1N HCl时,Zeta电位及电导率的变化。该过程是由美国MAS公司生产的超声电声法粒度及Zeta电位分析仪Zeta-APS设备完成的。图2显示,在pH<4时,Zeta曲线接近一个稳定区域,而电导率增加得更快。这表明,随着pH值的降低,二氧化硅颗粒逐渐被H+离子饱和。当更多的H+离子停留在连续的水溶剂中时,电导率增加得更快,而不是通过滑面向颗粒表面扩散。
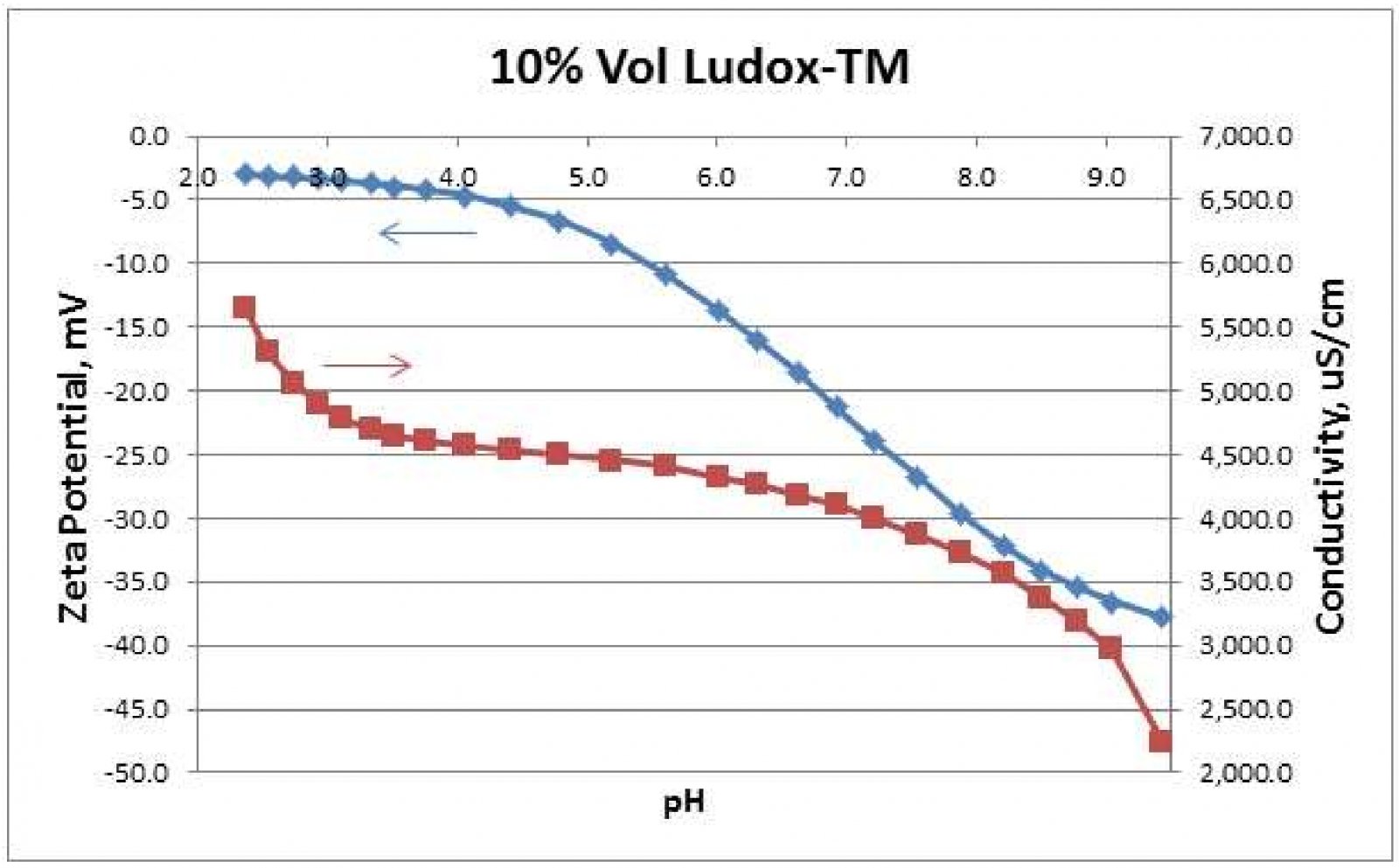
Figure 2. Automatic potentiometric titration of 10%-vol Ludox-TM by 1N HCl addition.
图2通过自动电位滴定法将1N HCl加入到10%体积的Ludox TM二氧化硅溶液中
5. 体积滴定法测定Zeta电位和添加剂的关系
The Zeta-APS instrument is also capable of performing automatic Volumetric titrations whereby a reagent such as a surfactant is added into a colloid in dosages as small as 1 μL. The Zeta-APS then produces titration graphs showing plots such as Zeta potential, pH, Conductivity, and Temperature vs. added reagent volume. Plots of Zeta vs. reagent volume would be flat if the added surfactant is not adsorbed by the particles, i.e. it does not supply potential-determining ions1-2.
Zeta-APS仪器还能够执行自动体积滴定,即将表面活性剂等试剂以1μL的剂量添加到胶体中。Zeta-APS随后生成滴定图,显示Zeta电位、pH值、电导率和温度与添加的试剂体积的关系图。如果添加的表面活性剂未被颗粒吸附,则它没有提供改变电位的粒子1-2,则Zeta与试剂体积的关系图将是稳定的。
References
1. Hunter, R. J., Introduction to Modern Colloid Science, Oxford Science Pub., 1993.
2. Morrison, I. D., Sydney, R., Colloidal Dispersions. Suspensions, Emulsions, and Foams. John Wiley and Sons, 2002.

